ICMRA HARMONISATION UPDATE
ICMRA HARMONISATION UPDATE
In June this year the TNHA broke the news of an international medicines regulatory harmonisation scheme being orchestrated behind closed doors by the heads of twenty international drug regulatory bodies, called the International Coalition of Medicines Regulatory Authorities, or ICMRA in short.
We are particularly concerned about this secretive organisation, following revelations about it having established a Complementary Medicine and Herbal Medicine Working Group which was first mooted by, and now chaired by the Australian Therapeutic Goods Administration (TGA). The first meeting of this working group took place in the Netherlands in 2014.
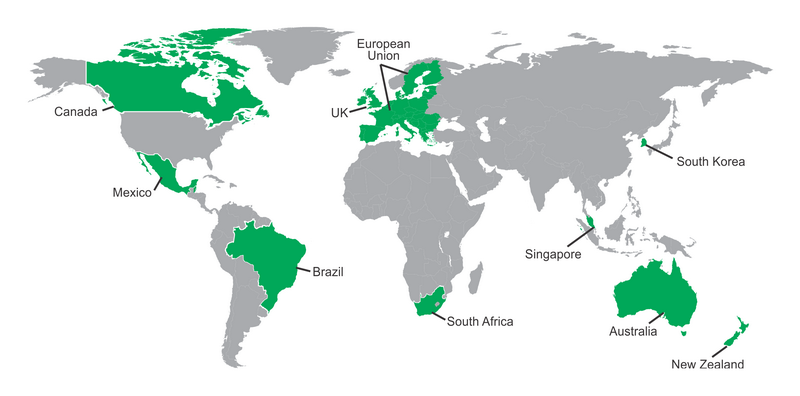
In a response to our written enquiry to the South African Medicines Control Council on the 20th of July, we have been informed that the regulatory heads of the following National Regulators participate in this working group.
- Australia – Therapeutic Goods Administration (TGA)
- Brazil – National Health Surveillance Agency (ANVISA)
- Canada – Health Products and Food Branch, Health Canada (HPFB-HC)
- Mexico – Federal Commission for the Protection against Sanitary Risks (COFEPRIS)
- European Union – European Medicines Agency (AMA)
- Singapore – Health Sciences Authority Singapore (HSA)
- Switzerland – Swissmedic
- South Africa – Medicines Control Council (MCC)
- United Kingdom – Medicines and Healthcare Products Regulatory Agency (MHRA)
- New Zealand – Medsafe, New Zealand Medicines and Medical Devices Safety
- Netherlands – Medicines Evaluation Board (MEB)
- South Korea – Ministry of Food and Drug Safety (MFDS)
The stated aim of this working group is to ‘share the best practices on the regulatory oversight on complementary medicines and herbal medicines‘.
Our Medicines Control Council had this to say about our country’s membership to ICMRA recently. “It is evident that globally, National Regulatory Authorities (NRAs) are developing harmonised regulatory frameworks rapidly and South Africa needs to follow that direction. The area of individual NRAs is declining and networks of regulators are getting more important. South African needs to reply more on harmonised approaches with out agencies and to increase its added value activities.”
Without any public or NGO participation allowed at these secretive meetings held twice a year in different locations around the world, we have no way of knowing what is being discussed and what level of consensus is being reached in attempting to implement a harmonised regulatory framework for Natural Health Products.
RUSSIA JOINS ICMRA
Although the ICMRA was first envisaged and initiated in 2011 by former U.S. Food and Drug Administrator Margaret Hamburg, it was only officially launched in 2013. It has until recently been running in an interim phase. As from next year (2017), ICMRA will expand it’s membership base to include other countries.
The last ICMRA meeting was held between the 11th and 13th of October in Switzerland.

The regulatory heads of the National Medicines Regulatory Authorities who attended the recent ICMRA meeting in Switzerland.
At this Swiss meeting the Federal Drug Control Service of the Russian Federation gained membership to ICMRA, bringing the number of member states represented to twenty-two.

Russia (in red) joined ICMRA in October 2016. The existing member States (in orange) joined in 2013.
NEW ICMRA LEADERSHIP
From its inception in October 2013 until October 2016 the Interim Secretariat of the ICMRA was chaired by Canada’s Health Products and Food Branch.
Ireland’s Health Product Regulatory Authority and the Ministry of Health, Labour and Welfare, and the Pharmaceuticals and Medical Devices Agency of Japan were interim Vice-Chairs.
At its last meeting in Switzerland, the new leadership was voted in.
